Overview: Implantable Kidneys
1. How does the device work? And how big is the device?
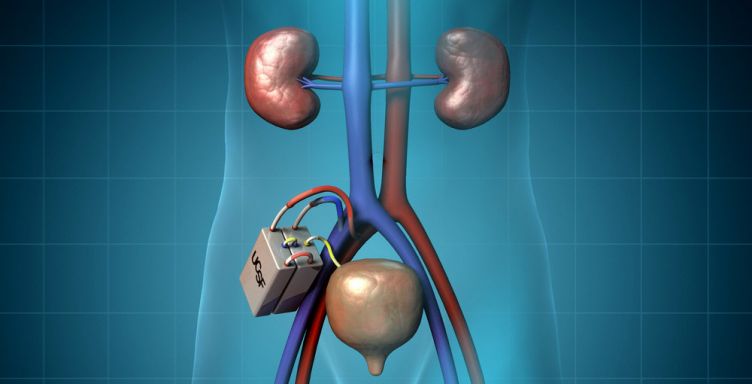
The bioartificial kidney, the size of a coffee cup, consists of two modules that work together to get rid of wastes. First, a hemofilter module processes incoming blood to create a watery ultrafiltrate that contains dissolved toxins as well as sugars and salts. Second, a bioreactor of kidney cells processes the ultrafiltrate and sends the sugars and salts back into the blood. In the process, water is also reabsorbed back into the body, concentrating the ultrafiltrate into “urine,” which will be directed to the bladder for excretion.
2. What is the surgical process like to insert the device? Where can I have the procedure done?
The procedure will be similar to kidney transplant surgery and it will be performed under general anesthesia. Once the bioartificial kidney device is available publicly, the procedure can be completed at any hospital with a trained transplant surgical team.
3. How will the filter be cleaned?
The filter is coated with a special thin biocompatible film to prevent fouling and blood clots. Additionally, the blood flowing over the coated filter surface will also help to keep the membrane free of debris accumulation.
4. How long will the device survive after implantation? Will it have to be replaced?
The device is meant to be permanent and that is what our efforts are pushing towards. Current testing and research suggest that it could be possible for the device to operate for many years, without failure. However, if failures occur, the replacement of the filter and/or cells would likely involve a minimally invasive surgery.
5. Will patients still have to take immunosuppressive drugs or anticoagulant after implantation? Will there be a risk of blood clotting?
The cells in the bioreactor are isolated from the patient’s immune system by the scaffold on which they are grown. The coatings on the device work to prevent blood clotting. In earlier human trials of a large scale bioartificial kidney, no antirejection drugs were needed, and in recent preclinical experiments, no blood thinners were needed.
6. How are the cells in the bioreactor simultaneously isolated from the immune system but still kept alive?
The immunoisolation is provided by the membranes on which the cells are grown. The cells are grown on a porous scaffold which allows water, salts, glucose, amino acids, and other very small molecules to pass through it freely. These nourish the cells and allow the cells to dispose of small wastes, such as carbon dioxide.
The immune system relies on fairly large molecules to identify and attach foreign intruders, which are a thousand times larger than, say, glucose. They are too large to penetrate the sieve of the membrane supporting the cells.
7. Will it also help Polycystic Kidney Disease and Focal Segmental Glomerulosclerosis (FSGS)?
Yes. In any case where a kidney transplant is needed, our device will be a viable option.
8. Will the “cell reactor” part of your kidney be able to generate Erythropoietin and eliminate the need for artificial EPO injections?
The kidney cells in the bioreactor are different cells than the cells that secrete erythropoetin.
9. What kind of GFR value would you expect to see once this device gets implanted in you? How much increase in GFR might occur?
While this will be confirmed as we progress through preclinical studies, we anticipate a GFR value of 20-30 ml/min with this device implanted.
10. Can you still get this device if you still have 15% kidney function? Or is it exclusive to those whose kidneys have totally failed?
This is case-specific, so it would depend on the details of a particular patient’s case and will be decided by both the patient and physician.
11. Will this be available internationally? Or will international patients have to go to United States for procedure?
Once the device is released commercially, it will be available to all patients in need, domestic or international. Clinical trials, however, will likely be conducted in the United States initially because it is where the key research is taking place as well as needing to adhere to all FDA guidelines.
12. Any predicted side effects?
Adverse side-effects that might occur would be similar to side effects known in other procedures involving implanted medical devices. These include complications like surgical trauma, scars, and infections. Additionally, there might be a need for increased fluid consumption.
13. How much will this device cost?
We anticipate that the device will cost no more than what transplant costs. It is challenging to put a dollar figure on procurement, implantation, and monitoring in the future due to what the business and economic climate will be at the time the device is released.
14. Will this device be covered by insurance?
Generally, medical devices that are successful are products for which insurance provides coverage. Our analysis suggests that the implantable bioartificial kidney will be associated with over 50% cost savings compared to dialysis and, as such, we anticipate that the device will be attractive to those considering coverage decisions.
15. What are the challenges (long term and short term)?
The Kidney Project is an ambitious project and is not without its challenges. In the short term, our primary challenge is funding the research and development. A significant hurdle is procuring enough money in order to complete the preclinical studies for both device components, and build full-scale prototypes for the first rounds of in-human studies.
The long term challenges center around keeping the device operating trouble-free after implantation beyond a few months. Some problems won’t become clear until we do clinical trials, so for now, we are looking at ways to increase the lifetime of cells as well as ways to minimize, or even eliminate, thrombus formation (blood clotting). We will get a better handle on the long-term challenges once we transition to preclinical studies and begin to gather clinical-scale data.
16. Why did The Kidney Project partner with Home Dialyzors United (HDU), and what does that partnership mean?
In spring of 2017, The Kidney Project announced a new partnership with Home Dialyzors United. We are very excited about the opportunities this partnership will bring, and believe it is an important milestone for The Kidney Project.
Partnerships with patient-advocacy groups, such as HDU, allow The Kidney Project to access the opinions and the unique perspectives of kidney disease patients, helping to improve the bioartificial kidney device for all users. The FDA (Food and Drug Administration) encourages medical device developers to incorporate patient perspectives, acknowledging that their voice is often unheard in the medical device development process.
HDU works tirelessly within today’s legal and medical systems to advocate for, and inform dialysis patients about their options for dialysis care. We have partnered with HDU because of their advocacy experience, and their deep understanding of the daily obstacles presented to dialysis patients, and their loved ones.
The Timeline
17. What can be done to speed up the research and development process?
Our progress is driven by two components: time required to conduct scientific analysis and development, and funding. The scientific analysis and development process cannot necessarily be forced into a faster pace. Because we are developing a novel device and are held to the safety standards of the FDA, we must maintain scientific rigor and technical excellence in a logical order without skipping steps.
However, additional funding allows us to hire more personnel, expedite our ordering, purchase additional equipment and generally complete tasks faster. Therefore, donations, fundraisers, and federal funding all help to speed up the research and development process. In short, more funds allow us to operate at a faster pace.
18. How long with clinical trials take to complete?
There are several required steps to the clinical trial process.
Our initial clinical trial, anticipated to begin by early 2018, will be a materials safety test of the Hemofilter. During this trial, we will confirm that all the materials included in our Hemofilter component are safe for human blood exposure. This is the first step of many to receive FDA approval for a completed bioartificial kidney device.
Once our initial clinical trial is complete and the data analyzed, we will apply for a follow-up study to test the Hemofilter’s functional components, namely its ability to filter human blood. Subsequent clinical tests will focus on verifying individual components of the Hemofilter until we graduate into full device implantation in humans. We anticipate this could occur as early as late 2018.
A truncated series of clinical trials will then be required for the combined Hemofilter and Bioreactor device, i.e. the bioartificial kidney. After these trials are completed, the bioartificial kidney will be available to the public. We expect to arrive at this final stage of clinical trials by 2020. This scenario assumes that we have sufficient funding and no unanticipated scientific, technical, or regulatory drawbacks
19. When will the device be ready after clinical trials?
See: How long will the clinical trials take to complete, above.
During the clinical trials, we will be working with manufacturers to discuss and manage the details of production.
Watch this UCSF informative video
NOTE: The information above was taken from UCSF’s School of Pharmacy and Medicine’s website at this link: https://pharm.ucsf.edu/kidney